On the rearrangements of biologically-relevant vinyl allene oxides to cis-cyclopentenones, ketols, and Favorskii-type carboxylic acids†
Abstract
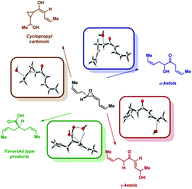
In addition to stereodefined cis-cyclopentenones, the rearrangement of naturally-occurring vinyl allene oxides can provide ketols, cyclopropylcarbinols, and Favorskii-type bis-(Z)-but-2-en-1-yl acetic acids. These processes have been studied by DFT computations using (Z)-but-1-en-1-yl allene oxides as model systems. Prior studies on the stepwise cascade process starting from (Z)-but-1-en-1-yl allene oxides established as key steps the ring opening of the oxirane to give oxidopentadienyl biradicals, and their isomerization through formation of alkenylcyclopropanone intermediates prior to the conrotatory electrocyclic ring closure to cis-configured cyclopentenones. Under neutral or under acidic conditions, the corresponding ketols and cyclopropylcarbinols have been computationally characterized as resulting from SN2, SN1 and SN1′-type processes, showing that the rearrangement of vinyl allene oxides is pH-dependent. Moreover, stereoconvergent base-induced Favorskii-type rearrangements to provide bis-(Z)-but-1-en-1-yl substituted acetic acids have also been justified. Since the model system captures the structural features of the vinyl allene oxides of biological relevance, our computations provide the most comprehensive overview of the complex reactivity of these natural species.
- This article is part of the themed collection: Mechanistic, computational & physical organic chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...