Chemical catalyst-promoted photooxygenation of amyloid proteins
Abstract
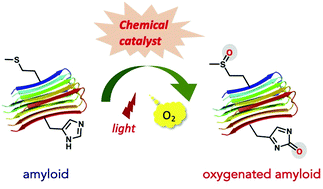
Misfolded proteins produce aberrant fibrillar aggregates, called amyloids, which contain cross-β-sheet higher order structures. The species generated in the aggregation process (i.e., oligomers, protofibrils, and fibrils) are cytotoxic and can cause various diseases. Interfering with the amyloid formation of proteins could be a drug development target for treating diseases caused by aberrant protein aggregation. In this review, we introduce a variety of chemical catalysts that oxygenate amyloid proteins under light irradiation using molecular oxygen as the oxygen atom donor (i.e., photooxygenation catalysts). Catalytic photooxygenation strongly inhibits the aggregation of amyloid proteins due to covalent installation of hydrophilic oxygen atoms and attenuates the neurotoxicity of the amyloid proteins. Recent in vivo studies in disease model animals using photooxygenation catalysts showed promising therapeutic effects, such as memory improvement and lifespan extension. Moreover, photooxygenation catalysts with new modes of action, including interference with the propagation of amyloid core seeds and enhancement in the metabolic clearance of amyloids in the brain, have begun to be identified. Manipulation of catalytic photooxygenation with secured amyloid selectivity is indispensable for minimizing the side effects in clinical application. Here we describe several strategies for designing catalysts that selectively photooxygenate amyloids without reacting with other non-amyloid biomolecules.


 Please wait while we load your content...
Please wait while we load your content...