K2S2O8-mediated coupling of 6-amino-7-aminomethyl-thiazolino-pyridones with aldehydes to construct amyloid affecting pyrimidine-fused thiazolino-2-pyridones†
Abstract
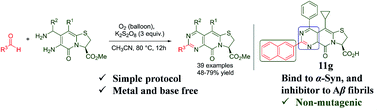
We herein present the synthesis of diversely functionalized pyrimidine fused thiazolino-2-pyridones via K2S2O8-mediated oxidative coupling of 6-amino-7-(aminomethyl)-thiazolino-2-pyridones with aldehydes. The developed protocol is mild, has wide substrate scope, and does not require transition metal catalyst or base. Some of the synthesized compounds have an ability to inhibit the formation of Amyloid-β fibrils associated with Alzheimer's disease, while others bind to mature amyloid-β and α-synuclein fibrils.


 Please wait while we load your content...
Please wait while we load your content...