Diastereoselective desymmetrization reactions of prochiral para-quinamines with cyclopropenes generated in situ: access to fused hydroindol-5-one scaffolds†
Abstract
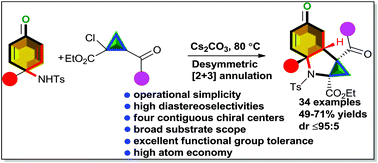
Interesting desymmetric [3 + 2] annulation reactions between p-quinamines as prochiral N-donors and 2-aroyl-1-chlorocyclopropanecarboxylates facilitated by a base are reported. This successive double Michael reaction delivered a unique class of cyclopropane-fused hydoindol-5-one frameworks, each having four contiguous stereogenic centers, with three of them being fully substituted. Moreover, this method was found to provide acceptable chemical yields with promising diastereoselectivities (dr of up to ≤95 : 5) and to work with a variety of substrates. Importantly, a polycyclic tacrine analogue used to treat Alzheimer's disease was synthesized using our developed method.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...