Synthesis of trisubstituted hydrazine via MnO2-promoted oxidative coupling of N,N-disubstituted hydrazine and boronic ester†
Abstract
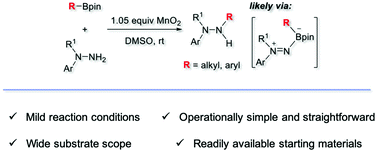
A MnO2-promoted oxidative coupling process between N,N-disubstituted hydrazine and boronic ester is reported. A 1,1-diazene species is firstly generated upon oxidation of a hydrazine substrate in the presence of MnO2 which then interacts with boronic ester to form the key intermediate boron-ate complex, followed by migration from boron to nitrogen to form a new C–N bond. This new finding provides mild, scalable, and operationally straightforward access to trisubstituted hydrazine.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...