Synthesis and biological activity of cyclopropyl Δ7-dafachronic acids as DAF-12 receptor ligands†
Abstract
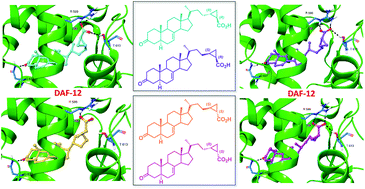
The four cyclopropyl stereoisomers of Δ7-dafachronic acids were prepared from the bile acid hyodeoxycholic acid and employed as chemical tools to exploit the importance of the orientation and spatial disposition of the carboxyl tail and the C25-methyl group for the binding at the DAF-12 receptor. The synthesis route was based on (a) Walden inversion and stereoselective PtO2-hydrogenation to convert the L-shaped 5β-cholanoid scaffold into the planar 5α-sterol intermediate; (b) two-carbon homologation of the side chain by Wittig and cyclopropanation reaction; and (c) formation of the 3-keto group and Δ7 double bond. The synthesized isomers were isolated and tested for their activity as DAF-12 ligands by AlphaScreen assays. Results showed a significant loss of potency and efficacy for all the four stereoisomers when compared to the parent endogenous ligand. Computational analysis has evidenced the configurational and conformational arrangement of both the carboxylic and the C25-methyl group of dafachronic acids as key structural determinants for DAF-12 binding and activation.
- This article is part of the themed collection: Chemical Biology in OBC


 Please wait while we load your content...
Please wait while we load your content...