Total synthesis of cynaropicrin†
Abstract
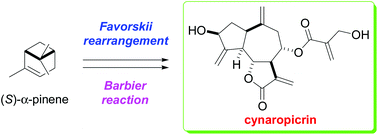
Cynaropicrin is found in artichoke (Cynara scolymus) and is the source of its bitter taste and it is a sesquiterpene lactone with a 5-7-5 tricyclic skeleton, six chiral centers, and four exo-olefins. This natural product has numerous attractive biological activities including the inhibition of NF-κB activation, antihepatitis C activity, and antitrypanosomal activity. In this study, the first total synthesis of cynaropicrin was achieved starting from (S)-α-pinene. The synthesis involved a stereoselective Favorskii rearrangement and an indium-promoted diastereoselective Barbier reaction.
- This article is part of the themed collection: Total synthesis in OBC


 Please wait while we load your content...
Please wait while we load your content...