Facile access to foldable redox-active flavin-peptide conjugates†
Abstract
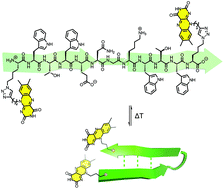
A convenient approach for the synthesis of foldable redox-active flavin peptide conjugates was established. A model β-hairpin oligopeptide motif was utilized to demonstrate that azidolysine side-chains are readily functionalised with an alkyne-bearing flavine derivative. The folding equilibrium of the peptide backbone as well as the redox behaviour of the flavin moieties remains intact after the conjugation.
- This article is part of the themed collections: Synthetic electrochemistry and Supramolecular chemistry in OBC


 Please wait while we load your content...
Please wait while we load your content...