Synthesis of fused 1,2-naphthoquinones with cytotoxic activity using a one-pot three-step reaction†
Abstract
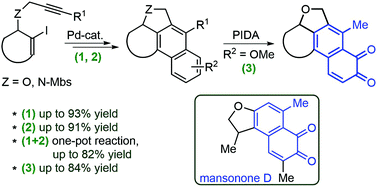
A method for the synthesis of fused 1,2-naphthoquinones, as analogues of biologically active natural terpene quinones, is described. The intermediate polycyclic naphthalenes were prepared by a one-pot palladium-catalysed process from simple alkynes, one of which was made from an optically pure biomass-derived levoglucosenone. The prepared methoxy-substituted naphthalenes were subsequently transformed in one step to 1,2-naphthoquinones by a trivalent-iodine-mediated oxidation. The naphthoquinone products were found to have cytotoxic properties.
- This article is part of the themed collection: Synthetic methodology in OBC

 Please wait while we load your content...
Please wait while we load your content...