Direct monitoring of biocatalytic deacetylation of amino acid substrates by 1H NMR reveals fine details of substrate specificity†
Abstract
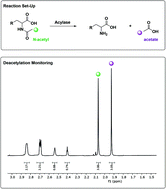
Amino acids are key synthetic building blocks that can be prepared in an enantiopure form by biocatalytic methods. We show that the L-selective ornithine deacetylase ArgE catalyses hydrolysis of a wide-range of N-acyl-amino acid substrates. This activity was revealed by 1H NMR spectroscopy that monitored the appearance of the well resolved signal of the acetate product. Furthermore, the assay was used to probe the subtle structural selectivity of the biocatalyst using a substrate that could adopt different rotameric conformations.


 Please wait while we load your content...
Please wait while we load your content...