Grafting chelating groups on 2D carbon for selective heavy metal adsorption†
Abstract
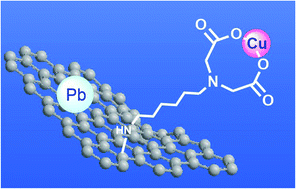
Iminodiacetic acid (IDA) is a tridentate ligand, which can capture metal ions by forming two fused five-membered chelate rings. In this study, we fixed IDA moieties onto a two-dimensional nanocarbon, graphene oxide (GO), to obtain materials with high and selective adsorption of metal ions. The synthesis conditions for the GO–IDA composites were optimized, then their structures were characterized by infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS), and CHN elemental analysis. In addition, the heavy-metal removal efficiency and selectivity of the GO–IDA composites with different length alkyl linkers between the GO and IDA were investigated. An aqueous solution containing 10 metal ions (Al, As, B, Cd, Cr, Cu, Mn, Pb, Se, and Zn) was used as a model for contaminated water at pH 7, and the interactions of the ions with GO–IDA were in the order of Cu > Pb > As > B > Zn > Al ≈ Se. The interaction between Cu and GO–IDA was confirmed by XPS and extended X-ray absorption fine structure (EXAFS), which showed that Cu was coordinated to IDA.


 Please wait while we load your content...
Please wait while we load your content...