The evolution of nucleosidic analogues: self-assembly of prodrugs into nanoparticles for cancer drug delivery
Abstract
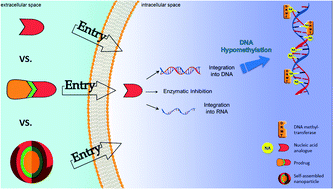
Nucleoside and nucleotide analogs are essential tools in our limited arsenal in the fight against cancer. However, these structures face severe drawbacks such as rapid plasma degradation or hydrophilicity, limiting their clinical application. Here, different aspects of nucleoside and nucleotide analogs have been exposed, while providing their shortcomings. Aiming to improve their fate in the body and combating their drawbacks, two different approaches have been discussed, the prodrug and nanocarrier technologies. Finally, a novel approach called “PUFAylation” based on both the prodrug and nanocarrier technologies has been introduced, promising to be the supreme method to create a novel nucleoside or nucleotide analog based formulation, with enhanced efficacy and highly reduced toxicity.
- This article is part of the themed collection: Recent Review Articles


 Please wait while we load your content...
Please wait while we load your content...