Formic acid dehydrogenation over single atom Pd-deposited carbon nanocones for hydrogen production: a mechanistic DFT study†
Abstract
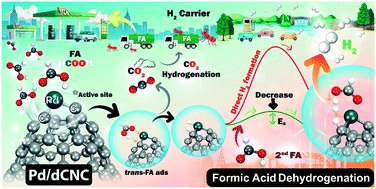
A single palladium (Pd) atom embedded in a high curvature defective carbon nanocone (Pd/dCNC) is investigated for formic acid (FA) decompositions using DFT calculations. We used Pd/dCNC as the catalyst for either trans-FA or cis-FA decomposition. FA is stably adsorbed on Pd/dCNC with very high adsorption energy compared to the Pd/dG surface. For the reaction mechanisms, the preferable FA dehydrogenation mechanisms proceed via the formate pathway, with a rate-determining step of only 0.50 eV (trans-FA) and 0.54 eV (cis-FA), which are less than that on active Pd (111) catalysts. The rate of hydrogen (H2) production is dependent on the FA concentration. The active neighboring C atom plays a significant role in facilitating FA dehydrogenation into H2. The side reaction producing CO and H2O via the formyl or carboxyl pathway cannot occur on Pd/dCNC due to a high energy-barrier and low production rate obtained by microkinetic simulations. Thus, our proposed catalysts effectively provide excellent activity and selectivity for FA dehydrogenation into H2.


 Please wait while we load your content...
Please wait while we load your content...