2-Difluoromethylpyridine as a bioisosteric replacement of pyridine-N-oxide: the case of quorum sensing inhibitors†
Abstract
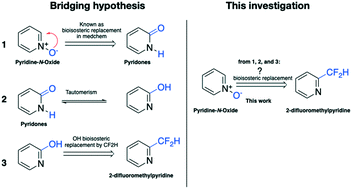
Herein, we demonstrate that 2-difluoromethylpyridine is a bioisosteric replacement of pyridine-N-oxide. Using the quorum sensing inhibitor 4NPO as a model compound, a library of 2-difluoromethylpyridine derivatives was designed, synthesized, and evaluated toward quorum sensing activity, biofilm formation, anti-violacein activity, and protease activity. As a result, compounds 1 (IC50 of 35 ± 1.12 μM), 5 (IC50 of 19 ± 1.01 μM), and 6 (IC50 of 27 ± 0.67 μM) showed a similar or better activity in comparison to 4NPO (IC50 of 33 ± 1.12 μM) in a quorum sensing system of Pseudomonas aeruginosa. In addition, compounds 1, 5, 6, and 4NPO showed good antibiofilm biomass of Pseudomonas aeruginosa and reduced violacein production in Chromobacterium violaceum. In terms of protease activity, compounds 1, 5, and 6 showed significant activity compared to 4NPO. Overall, the replacement of pyridine-N-oxide by 2-difluoromethylpyridine enhances the activity of the model compound, which could open a new path for bioisosteric replacement in drug discovery and development.


 Please wait while we load your content...
Please wait while we load your content...