Reactivity of N-acyl hydrazone probes with the mammalian proteome†
Abstract
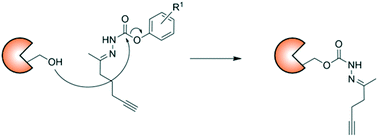
Small molecule probes with distinct reactivities are useful tools for the identification and characterization of protein modifications and function. Herein, we show that hydrazone probes with an N-carbamate structural motif react differently from N-carbamates within the human proteome. Mass spectrometry analysis of probe-treated mammalian cell lysates identified several proteins that were covalently modified by the hydrazone probes, including the cytidine deaminase APOBEC3A. We used this enzyme as a model to explore the reactivity of the probes with amino acid residues using LC–MS/MS. Both reactive serine and cysteine residues outside of the enzyme active site were covalently modified. A 1-napthol leaving group provided the most extensive reactivity. These results confirm a unique chemotype for hydrazone probes which can be further optimized to target distinct targets of the human proteome.

 Please wait while we load your content...
Please wait while we load your content...