Full-colour solvatochromic fluorescence emitted from a semi-aromatic imide compound based on ESIPT and anion formation†
Abstract
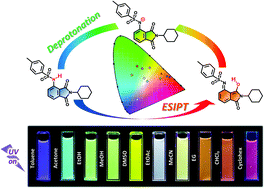
A multi-colour solvatochromic fluorophore was developed by substituting a fully organic 3-amino-N-cyclohexyl phthalimide group with a p-toluenesulfonyl (tosyl) group. This compound is essentially colourless in the visible region and exhibits reddish fluorescence through excited-state intramolecular proton transfer (ESIPT) in the crystalline state with a considerably large Stokes shift of 9786 cm−1, as well as bright fluorescence in the full-colour range, from purple to red, when dissolved in 10 different organic solvents. Excitation wavelength measurements, lifetime measurements, and time-dependent density functional theory (TD-DFT) calculations clarified that the full-colour solvatochromism of the fluorescence results from the combination of the fluorescence emissions from the three different excited states: locally excited (N*), intramolecular proton-transferred (T*), and anion states (A*). This unique solvatochromic fluorescence property is expected to be applied in organic electronic devices and biological imaging tools.


 Please wait while we load your content...
Please wait while we load your content...