The influence of alkyl group regiochemistry and backbone fluorination on the packing and transistor performance of N-cyanoimine functionalised indacenodithiophenes†
Abstract
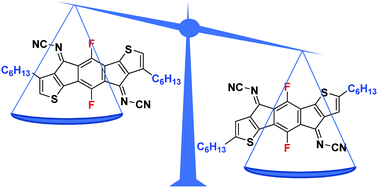
The synthesis of two novel n-type molecular organic semiconductors based on a fluorinated indacenodithiophene core in combination with an electron withdrawing N-cyanoimine group is reported, and the influence of the regiochemistry of the solubilizing sidechain is investigated. The N-cyanoimine is confirmed to be a strongly electron accepting group, which in combination with the core fluorination resulted in high electron affinities for both materials. Single crystal analysis demonstrated that whilst both materials arrange in ordered slipped stacks with close π–π stacking distances (∼3.40 Å), significant differences in electron transfer integrals for the two regioisomers were observed, relating to differences in relative molecular displacement along the π-stacking direction. Organic thin-film transistors fabricated via blade-coating displayed electron mobility up to 0.13 cm2 V−1 s−1 for the isomer with the larger transfer integral.


 Please wait while we load your content...
Please wait while we load your content...