De-glycosylation and enhanced bioactivity of flavonoids from apple pomace during extraction with deep eutectic solvents†
Abstract
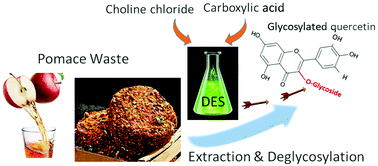
Deep eutectic solvents (DES) are attracting great attention as a green alternative to hazardous organic solvents for the extraction of valuable bioactive compounds from agricultural and processing waste. In this study, the simultaneous extraction and de-glycosylation of quercetin derivatives from apple pomace was achieved by using various DES made of choline chloride and different types of carboxylic acids. The content of quercetin derivatives was similar in DES and conventional methanol extracts, but their relative amounts were distinctively different due to de-glycosylation during DES extraction. Chromatographic and spectroscopic analyses allowed the determination of the structures of the glycosylated quercetins, and revealed that the proportion of the quercetin aglycon in DES extracts can be controlled by the DES composition, extraction temperature and duration of extraction. Of all quercetin glycoconjugates present, quercetin-3-O-α-arabinofuranoside was more prone to de-glycosylation during DES extraction than any other quercetin derivative. Furthermore, the antioxidant activity of the DES extracts was higher than that of methanol extracts, likely due to the increase of quercetin aglycon content during extraction. These data show the potential to extract and de-glycosylate quercetin derivatives in a single step using green DES to generate bioactive preparations with enhanced activity.


 Please wait while we load your content...
Please wait while we load your content...