Photoredox synthesis of functionalized quinazolines via copper-catalyzed aerobic oxidative Csp2–H annulation of amidines with terminal alkynes†
Abstract
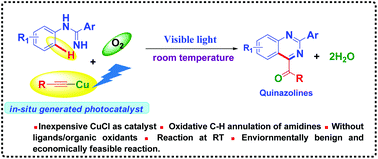
We have developed a visible light-induced photo-redox copper-catalyzed oxidative Csp2–H annulation (Friedel–Crafts-type cyclization) of amidines with terminal alkynes at room temperature to synthesize functionalized quinazolines. We report copper(I)-phenylacetylide catalyzed photo-oxidative Csp2–H annulation of amidines at RT, which is very challenging and complementary to the conventional transition metal-catalyzed thermal annulation reactions. We have demonstrated the application of this method by synthesizing anti-cancer compounds. Moreover, green chemistry metrics (the E-factor is ∼1.9 times better than that of the reported thermal method) and Eco-Scale (scales 55.4, which shows an acceptable synthesis) evaluations show that this method is eco-friendly and environmentally feasible.


 Please wait while we load your content...
Please wait while we load your content...