Poly(methylhydrosiloxane) as a reductant in the catalytic base-free Wittig reaction†
Abstract
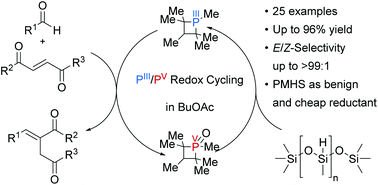
Herein, we report a catalytic, base-free Wittig reaction forming highly functionalized alkenes with PMHS as a terminal reductant and butylacetate as a green solvent. Poly(methylhydrosiloxane) (PMHS) is a non-toxic, enviromentally friendly, inexpensive and easy to handle reductant. However, the inherent low reactivity hampers its applicability in catalytic reactions, such as P(III)/P(V) redox cycling reactions. Most of these catalytic systems include highly active aryl silanes to facilitate phosphane oxide reduction and are not compatible with PMHS or similar more sustainable terminal reductants. The herein reported catalyst system which is based on a methyl-substituted phosphetane operates at low catalyst loadings without additional co-catalysts and allowes the use of PMHS as terminal reductant. A wide variety of functional groups was tolerated and 25 different alkenes were synthesized in yields up to 96% with excellent stereoselectivity. Mechanistic studies revealed the formation of water from silanol condensation as the main pathway of siloxane formation.


 Please wait while we load your content...
Please wait while we load your content...