Mizoroki–Heck type reactions and synthesis of 1,4-dicarbonyl compounds by heterogeneous organic semiconductor photocatalysis†
Abstract
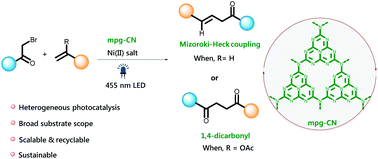
We report the synthesis of 1,4-dicarbonyl compounds and substituted alkenes (Mizoroki–Heck type coupling) starting from secondary and tertiary alkyl halides and vinyl acetate or styrene derivatives using visible-light photocatalysis. The protocol uses mesoporous graphitic carbon nitride (mpg-CN) as a heterogeneous organic semiconductor photocatalyst and Ni(II) salts as Lewis acid catalysts. Detailed post-characterization of the heterogeneous material has been carried out to support the proposed catalytic cycle. Apart from high functional-group tolerance, mild reaction conditions, scalability as well as easy recovery and reuse of the mpg-CN photocatalyst provide a practical solution to these widespread transformations in terms of sustainability and efficiency and this methodology is recommended for applications in academic and industrial synthesis.
- This article is part of the themed collection: 2021 Green Chemistry Hot Articles


 Please wait while we load your content...
Please wait while we load your content...