The fate of poly- and perfluoroalkyl substances in a marine food web influenced by land-based sources in the Norwegian Arctic†
Abstract
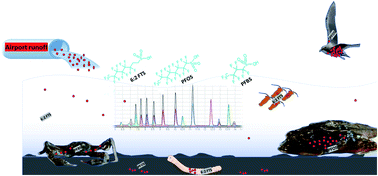
Although poly- and perfluorinated alkyl substances (PFAS) are ubiquitous in the Arctic, their sources and fate in Arctic marine environments remain unclear. Herein, abiotic media (water, snow, and sediment) and biotic media (plankton, benthic organisms, fish, crab, and glaucous gull) were sampled to study PFAS uptake and fate in the marine food web of an Arctic Fjord in the vicinity of Longyearbyen (Svalbard, Norwegian Arctic). Samples were collected from locations impacted by a firefighting training site (FFTS) and a landfill as well as from a reference site. Mean 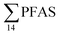 concentration in the landfill leachate was 643 ± 84 ng L−1, while it was 365 ± 8.0 ng L−1 in a freshwater pond and 57 ± 4.0 ng L−1 in a creek in the vicinity of the FFTS. These levels were an order of magnitude higher than in coastal seawater of the nearby fjord (maximum level
concentration in the landfill leachate was 643 ± 84 ng L−1, while it was 365 ± 8.0 ng L−1 in a freshwater pond and 57 ± 4.0 ng L−1 in a creek in the vicinity of the FFTS. These levels were an order of magnitude higher than in coastal seawater of the nearby fjord (maximum level 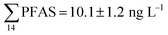 , at the FFTS impacted site). PFOS was the most predominant compound in all seawater samples and in freshly fallen snow (63–93% of
, at the FFTS impacted site). PFOS was the most predominant compound in all seawater samples and in freshly fallen snow (63–93% of  ). In freshwater samples from the Longyear river and the reference site, PFCA ≤ C9 were the predominant PFAS (37–59%), indicating that both local point sources and diffuse sources contributed to the exposure of the marine food web in the fjord.
). In freshwater samples from the Longyear river and the reference site, PFCA ≤ C9 were the predominant PFAS (37–59%), indicating that both local point sources and diffuse sources contributed to the exposure of the marine food web in the fjord. 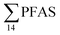 concentrations increased from zooplankton (1.1 ± 0.32 μg kg−1 ww) to polychaete (2.8 ± 0.80 μg kg−1 ww), crab (2.9 ± 0.70 μg kg−1 ww whole-body), fish liver (5.4 ± 0.87 μg kg−1 ww), and gull liver (62.2 ± 11.2 μg kg−1). PFAS profiles changed with increasing trophic level from a large contribution of 6:2 FTS, FOSA and long-chained PFCA in zooplankton and polychaetes to being dominated by linear PFOS in fish and gull liver. The PFOS isomer profile (branched versus linear) in the active FFTS and landfill was similar to historical ECF PFOS. A similar isomer profile was observed in seawater, indicating major contribution from local sources. However, a PFOS isomer profile enriched by the linear isomer was observed in other media (sediment and biota). Substitutes for PFOS, namely 6:2 FTS and PFBS, showed bioaccumulation potential in marine invertebrates. However, these compounds were not found in organisms at higher trophic levels.
concentrations increased from zooplankton (1.1 ± 0.32 μg kg−1 ww) to polychaete (2.8 ± 0.80 μg kg−1 ww), crab (2.9 ± 0.70 μg kg−1 ww whole-body), fish liver (5.4 ± 0.87 μg kg−1 ww), and gull liver (62.2 ± 11.2 μg kg−1). PFAS profiles changed with increasing trophic level from a large contribution of 6:2 FTS, FOSA and long-chained PFCA in zooplankton and polychaetes to being dominated by linear PFOS in fish and gull liver. The PFOS isomer profile (branched versus linear) in the active FFTS and landfill was similar to historical ECF PFOS. A similar isomer profile was observed in seawater, indicating major contribution from local sources. However, a PFOS isomer profile enriched by the linear isomer was observed in other media (sediment and biota). Substitutes for PFOS, namely 6:2 FTS and PFBS, showed bioaccumulation potential in marine invertebrates. However, these compounds were not found in organisms at higher trophic levels.
- This article is part of the themed collections: Recent Open Access Articles and Best Papers 2021 - Environmental Science: Processes & Impacts


 Please wait while we load your content...
Please wait while we load your content...