Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through production of sodium carbonate†
Abstract
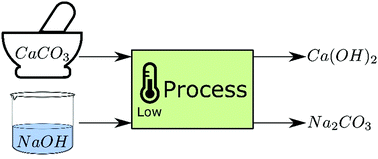
The calcination of calcium carbonate (CaCO3) is a major contributor to carbon dioxide (CO2) emissions that are changing our climate. Moreover, the calcination process requires high temperatures (∼900 °C). A novel low-temperature process for the decarbonisation of CaCO3 is tested whereby the CO2 is directly sequestered/mineralised in sodium carbonate. CaCO3 is reacted with an aqueous sodium hydroxide solution by mixing under atmospheric temperatures and pressures. The reaction products are calcium hydroxide (hydrated lime; Ca(OH)2) and sodium carbonate (soda ash; Na2CO3). For the first time, the extent of this reaction at ambient conditions is studied along with the NaOH requirements. Conceptual process designs, which include procedures to separate and recover material, as well as energy calculations, are also presented to demonstrate the technical/industrial feasibility of the process. The technology is also successfully tested on industrially sourced limestone chalk, and the silica impurity remains inert throughout the process. This technology will enable industrial symbiosis by combining the high-temperature lime and sodium carbonate manufacturing processes into a single low-temperature process and greatly reduce the chemical (raw material) CO2 emissions associated with the production of cement and lime.


 Please wait while we load your content...
Please wait while we load your content...