Exogenous electricity flowing through cyanobacterial photosystem I drives CO2 valorization with high energy efficiency†
Abstract
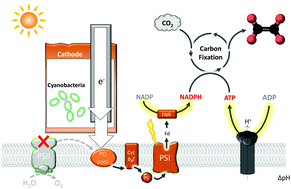
Nature's biocatalytic processes are driven by photosynthesis, whereby photosystems I and II are connected in series for light-stimulated generation of fuel products or electricity. Externally supplying electricity directly to the photosynthetic electron transfer chain (PETC) has numerous potential benefits, although strategies for achieving this goal have remained elusive. Here we report an integrated photo-electrochemical architecture which shuttles electrons directly to PETC in living cyanobacteria. The cathode of this architecture electrochemically interfaces with cyanobacterial cells that have a lack of photosystem II activity and cannot perform photosynthesis independently. Illumination of the cathode channels electrons from an external circuit to intracellular PETC through photosystem I, ultimately fueling cyanobacterial conversion of CO2 into acetate. We observed acetate formation when supplying both illumination and exogenous electrons under intermittent conditions (e.g., in a 30 s supply plus 30 min interval condition of both light and exogenous electrons). The energy conversion efficiency for acetate production under programmed intermittent LED illumination (400–700 nm) and exogenous electron supply reached ca. 9%, when taking into account the number of photons and electrons received by the biotic system, and ca. 3% for total photons and electrons supplied to the cyanobacteria. This approach is applicable for generating various CO2 reduction products by using engineered cyanobacteria, one of which has enabled electrophototrophic production of ethylene, a broadly used hydrocarbon in the chemical industry. The resulting bio-electrochemical hybrid has the potential to produce fuel chemicals with numerous potential advantages over standalone natural and artificial photosynthetic approaches.


 Please wait while we load your content...
Please wait while we load your content...