Role of intramolecular hydrogen bonding in the redox chemistry of hydroxybenzoate-bridged paddlewheel diruthenium(ii,ii) complexes†
Abstract
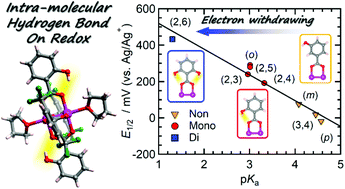
The synthesis of a series of trans-heteroleptic paddlewheel diruthenium(II,II) complexes with various hydroxy-substituted benzoate ligands, [Ru2((OH)xPhCO2)2(2,6-(CF3)2PhCO2)2(THF)2] ([RuII,II2]) as tetrahydrofuran (THF) adducts is reported, where (OH)xPhCO2− stands for o-hydroxybenzoate (o-OH), m-hydroxybenzoate (m-OH), p-hydroxybenzoate (p-OH), 2,3-dihydroxybenzoate (2,3-(OH)2), 2,4-dihydroxybenzoate (2,4-(OH)2), 2,5-dihydroxybenzoate (2,5-(OH)2), 2,6-dihydroxybenzoate (2,6-(OH)2), or 3,4-dihydroxybenzoate (3,4-(OH)2), and 2,6-(CF3)2PhCO2− represents 2,6-bis(trifluoromethyl)benzoate. In this heteroleptic series, the redox potential (E1/2) of the [RuII,II2]/[RuII,III2]+ couple in THF varies over a wide range, from −18 mV (vs. Ag/Ag+) for p-OH to 432 mV for 2,6-(OH)2. The redox properties are linearly dependent on the acidity (pKa) of the OH-substituted benzoic acids, but do not depend on the number of ortho-substituted hydroxy (o-OH) groups. This indicates that the steric effect of o-substituents is irrelevant in the case of hydroxyl groups, owing to the formation of intramolecular hydrogen bonds between the o-OH group and carboxylate oxygens. The value of the Hammett constant σo for the o-OH substituent was determined to be 0.667, indicating a strongly electron-withdrawing character, contrary to the expectation of electron-donating character for an OH group. The redox properties of the compounds were well explained in a framework of Hammett analyses and were also consistent with their HOMO energy levels evaluated by DFT calculations based on the atomic coordinates. The unexpected electron-withdrawing character of the o-OH groups could be attributed to the direct effect of intramolecular hydrogen bonding on the charge density on the carboxylate oxygen.


 Please wait while we load your content...
Please wait while we load your content...