Rationalizing the influence of α-cationic phospholes on π-catalysis†
Abstract
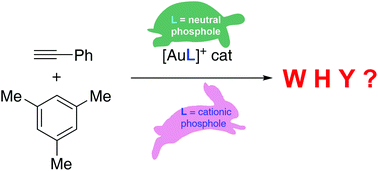
The physical factors behind the experimentally observed high activity of gold(I)-catalysts having an α-cationic phosphole as a ligand have been computationally explored. To this end, the gold(I)-catalysed hydroarylation reactions of phenylacetylene and mesitylene involving both neutral and cationic phosphole as well as phosphine ligands have been quantitatively analyzed in detail with the help of the activation strain model of reactivity in combination with the energy decomposition analysis method. It is found that the cationic phosphole ligands induce a dramatic change in both the geometry and the electronic structure of the initially formed π-complex which significantly enhances its electrophilicity. This results in an enhancement of the key π(mesitylene) → π*(LAu-acetylene complex) molecular orbital interaction which is the main factor responsible for the activating effect of these cationic ligands.


 Please wait while we load your content...
Please wait while we load your content...