Computational determination of the mechanism of the Pd-catalyzed formation of isatoic anhydrides from o-haloanilines, CO, and CO2†
Abstract
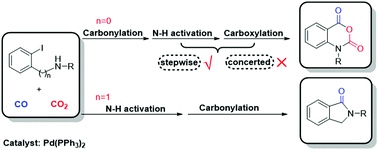
The palladium-catalyzed annulation of o-haloanilines with carbon monoxide (CO) and carbon dioxide (CO2), discovered by Wen-Zhen Zhang and co-workers, provides a convenient method to synthesize isatoic anhydrides. We explored the mechanism of this reaction, particularly the order of the reaction of CO and CO2 and the effect of the base, using density functional theory (DFT) calculations (ωB97X-D and M06). It was found that the base-assisted N–H bond activation through a concerted metalation–deprotonation (CMD) mechanism is a requisite for carboxylation, and the carboxylation proceeds via the nucleophilic attack of the (Pd)NH nitrogen on CO2. The results show that carbonylation occurs prior to carboxylation, because the facile and exergonic carbonylation greatly decreases the energies of the following intermediates and transition states. The mechanistic exploration of the alternative pathways (e.g., mono-carbonylation and carboxylation) and the comparison with the annulation mechanism of the o-iodobenzylamine substrate further demonstrate the perfect cooperation of CO and CO2 in constructing an anhydride moiety for o-haloanilines.


 Please wait while we load your content...
Please wait while we load your content...