Iron hexamesityl-5,15-diazaporphyrin: synthesis, structure and catalytic use for direct oxidation of sp3 C–H bonds†
Abstract
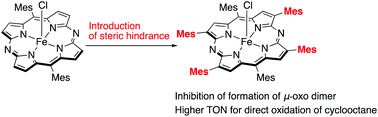
Iron hexamesityl-5,15-diazaporphyrin was synthesized through the cross-coupling reaction of tetrabromodiazaporphyrin. The use of chloroiron(III) hexamesityl-5,15-diazaporphyrin as a catalyst for oxidation of cyclooctane showed high performance with a total TON up to 731. The introduction of bulky mesityl groups at β-positions prevented the catalyst deactivation via formation of a μ-oxo dimer.


 Please wait while we load your content...
Please wait while we load your content...