A new generation of terminal copper nitrenes and their application in aromatic C–H amination reactions†
Abstract
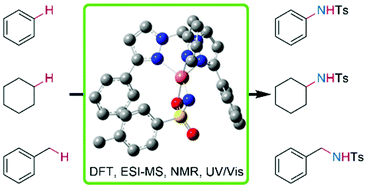
Copper nitrene complexes are highly reactive species and are known as intermediates in the copper catalyzed C–H amination. In this study, three novel copper tosyl nitrene complexes were synthesized at low temperatures, stabilized with heteroscorpionate ligands of the bis(pyrazolyl)methane family. The copper nitrenes were obtained by the reaction of a copper(I) acetonitrile complex with SPhINTs in dichloromethane. We show that the ligand design has a major influence on the catalytic activity and the thermal stability of the copper nitrene complex. Not only the choice of the third N donor, but also the substituent in the 5-position of the pyrazolyl moiety, have an impact on the stability. Furthermore, the novel copper nitrene complexes were used for catalytic aziridination of styrenes and C–H amination reactions of aromatic and aliphatic substrates under mild reaction conditions. Even challenging substrates like benzene and cyclohexane were aminated with good yields. The copper nitrene complexes were characterized using UV/Vis spectroscopy, low temperature Evans NMR spectroscopy, density functional theory, domain-based local pair natural orbital coupled cluster calculations (DLPNO-CCSD(T)) and cryo-UHR mass spectrometry.


 Please wait while we load your content...
Please wait while we load your content...