Hydrogen bonding to metals as a probe for an inverted ligand field†
Abstract
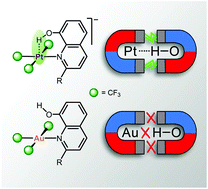
Electron-rich, late transition metals are known to act as hydrogen-bonding (HBd) acceptors. In this regard, Pt(II) centres in square-planar environments are particularly efficient. It is however puzzling that no convincing experimental evidence is currently available for the isoelectronic neighbour Au(III) being involved in HBd interactions. We report now on the synthesis and characterisation of two series of isoleptic and isoelectronic (d8) compounds [(CF3)3Pt(L)]− and (CF3)3Au(L), where the L ligands are based on the quinoline frame and have been selected to favour HBd with the metal centre. Strong HBd interactions were actually found in the Pt(II) compounds, based on structural and spectroscopic evidence, and they were further confirmed by theoretical calculations. In contrast, no evidence was obtained in the Au(III) case. In order to find the reason underlying this general disparity, we undertook a detailed theoretical analysis of the model systems [(CF3)3Pt(py)]− and (CF3)3Au(py). This study revealed that the filled dz2 orbital is the HOMO in the case of Pt(II), but is buried in the lower energy levels in the case of Au(III). The sharply different electronic configurations involve ligand-field inversion on going from Pt to the next element Au. This is not a gradual but an abrupt change, which invalidates Au(III) as a HBd-acceptor wherever ligand-field inversion occurs.


 Please wait while we load your content...
Please wait while we load your content...