Mechanism of methanol synthesis on Ni(110)†
Abstract
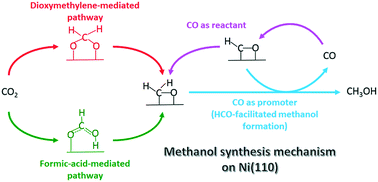
Planewave density functional theory (DFT-PW91) calculations are employed to study the methanol synthesis through CO2 and CO hydrogenation, as well as the two side reactions: the water gas shift (WGS) reaction and formic acid formation, on Ni(110). For the WGS reaction on Ni(110), we find that the redox mechanism is favored over the carboxyl-mediated mechanism. We show that the formate pathway is the dominant one for formic acid formation. For methanol synthesis through CO2 and CO hydrogenation on Ni(110), our results reveal that the formic-acid- and dioxymethylene-mediated pathways coexist, in contrast to methanol synthesis on Cu(111) where the formic-acid-mediated pathway dominates. We also find that on Ni(110), hydrogenation of CH2O* to CH3O* and that to CH2OH* both contribute to MeOH synthesis. Based on the derived energetics, we ascertain that CH3O* hydrogenation to CH3OH* is likely the rate-determining step along the CH3O* pathway on Ni(110). Remarkably, CH3O* hydrogenation can be facilitated by the presence of HCO*, demonstrating the promotional effect of CO. We further show that CO also participates in methanol synthesis directly via its hydrogenation to HCO* and further to CH2O*. Additional microkinetic modeling by considering feed composition and reaction conditions would provide further mechanistic insights into methanol synthesis on Ni(110).


 Please wait while we load your content...
Please wait while we load your content...