A new clade of styrene monooxygenases for (R)-selective epoxidation†
Abstract
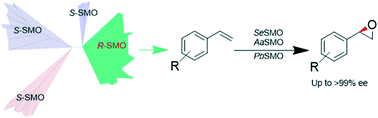
Styrene monooxygenases (SMOs) are excellent enzymes for the production of (S)-enantiopure epoxides, but so far, only one (R)-selective SMO has been identified with a narrow substrate spectrum. Mining the NCBI non-redundant protein sequences returned a new distinct clade of (R)-selective SMOs. Among them, SeStyA from Streptomyces exfoliatus, AaStyA from Amycolatopsis albispora, and PbStyA from Pseudonocardiaceae were carefully characterized and found to convert a spectrum of styrene analogues into the corresponding (R)-epoxides with up to >99% ee. Moreover, site 46 (AaStyA numbering) was identified as a critical residue that affects the enantioselectivity of SMOs. Phenylalanine at site 46 was required for the (R)-selective SMO to endow excellent enantioselectivity. The identification of new (R)-selective SMOs would add a valuable green alternative to the synthetic tool box for the synthesis of enantiopure (R)-epoxides.


 Please wait while we load your content...
Please wait while we load your content...