Electrochemical reduction of Cr(vi) in water: lessons learned from fundamental studies and applications
Abstract
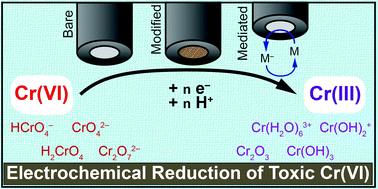
Converting toxic Cr(VI) to benign Cr(III) would offer a solution to decontaminate drinking water. Electrochemical methods are ideally suited to carry out this reduction without added external reductants. Achieving this transformation at low overpotentials requires mediating the transfer of protons and electrons to Cr(VI). In this review thermodynamic parameters will be discussed to understand Cr(VI) speciation in water and identify reduction pathways. The electrochemical reduction of Cr(VI) at bare electrodes is reviewed and mechanistic considerations are discussed. Works on modified electrodes are compared to identify key parameters influencing the reduction. An overview of current applications to Cr(VI) reduction is briefly discussed to link fundamental studies to applications.
- This article is part of the themed collection: 2020 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...