Thermodynamic properties of a molecular dipolar liquid using the two-phase thermodynamic approach
Abstract
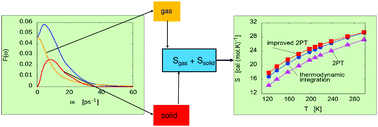
A modified version of the original two-phase thermodynamic approach has been extended to evaluate the thermodynamic properties of molecular systems. Its basic assumption states that the density of states can be decomposed into solid-like and gas-like components. The solid part has been approximated by that of a set of harmonic oscillators, whereas a subset composed of rough hard spheres has been considered for the gas part. In this new approach, molecules have been modelled as rotating hard spheres that experience elastic collisions. The technique has been tested on a system made up of dipolar diatomic molecules, and it leads to very good results for total entropy, potential energy reference and heat capacity. Translation and rotation solid components of the overall spectra have been compared to the real part of the instantaneous normal mode vibrational densities of states. Similarities between them reinforce the validity of the two-phase thermodynamic approach.


 Please wait while we load your content...
Please wait while we load your content...