Influence of the terminal group on the thermal decomposition reactions of carboxylic acids on copper: nature of the carbonaceous film
Abstract
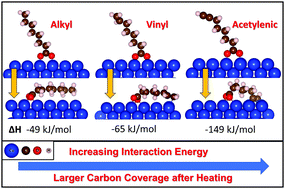
The effect of the terminal groups on the nature of the films formed by the thermal decomposition of carboxylic acids on copper is studied in ultrahigh vacuum using temperature-programmed desorption (TPD), scanning tunneling microscopy (STM) and Auger electron spectroscopy (AES). The influence of the presence of vinyl or alkynyl terminal groups and chain length is studied using heptanoic, octanoic, 6-heptenoic, 7-octenoic, 6-heptynoic and 7-octynoic acids. The carboxylic acids form strongly bound carboxylates following adsorption on copper at room temperature, and thermally decompose between ∼500 and 650 K. Previous work has shown that this occurs by the carboxylate plane tilting towards the surface to eliminate carbon dioxide and deposit a hydrocarbon fragment. The fragment can react to evolve hydrogen or form oligomeric species on the surface, where the amount of carbon increases for carboxylic acids that contain terminal functional groups that can anchor to the surface. These results will be used to compare with the carbonaceous films formed by the mechanochemical decomposition of carboxylic acids on copper, which occurs at room temperature. This is expected to lead to less carbon being deposited on the surface than during thermal decomposition.


 Please wait while we load your content...
Please wait while we load your content...
