Ultrafast geometrical reorganization of a methane cation upon sudden ionization: an isotope effect on electronic non-equilibrium quantum dynamics†
Abstract
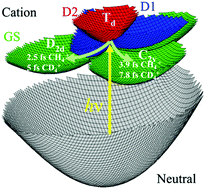
The ultrafast structural, Jahn–Teller (JT) driven, electronic coherence mediated quantum dynamics in the CH4+ and CD4+ cations that follows sudden ionization using an XUV attopulse exhibits a strong isotope effect. The JT effect makes the methane cation unstable in the Td geometry of the neutral molecule. Upon the sudden ionization the cation is produced in a coherent superposition of three electronic states that are strongly coupled and neither is in equilibrium with the nuclei. In the ground state of the cation the few femtosecond structural rearrangement leads first to a geometrically less distorted D2d minimum followed by a geometrical reorganization to a shallow C2v minimum. The dynamics is computed for an ensemble of 8000 ions randomly oriented with respect to the polarization of the XUV pulse. The ratio, about 3, of the CD4+ to CH4+ autocorrelation functions, is in agreement with experimental measurements of high harmonic spectra. The high value of the ratio is attributed to the faster electronic coherence dynamics in CH4+.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...