Mechanism and kinetics of the oxidation of 1,3-butadien-1-yl (n-C4H5): a theoretical study†
Abstract
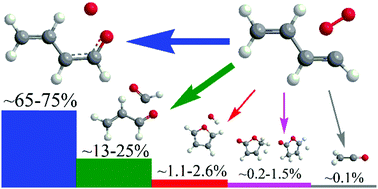
Ab initio CCSD(T)-F12/cc-pVTZ-f12//B3LYP/6-311G(d,p) calculations of the C4H5O2 potential energy surface have been combined with Rice–Ramsperger–Kassel–Marcus Master Equation (RRKM-ME) calculations of temperature- and pressure-dependent rate constants and product branching ratios to unravel the mechanism and kinetics of the n-C4H5 + O2 reaction. The results indicate that the reaction is fast, with the total rate constant being in the range of 3.4–5.6 × 10−11 cm3 molecule−1 s−1. The main products include 1-oxo-n-butadienyl + O and acrolein + HCO, with their cumulative yield exceeding 90% at temperatures above 1500 K. Two conformers of 1-oxo-n-butadienyl + O are formed via a simple mechanism of O2 addition to the radical site of n-C4H5 followed by the cleavage of the O–O bond proceeding via a van der Waals C4H5O⋯O complex. Alternatively, the pathways leading to acrolein + HCO involve significant reorganization of the heavy-atom skeleton either via formal migration of one O atom to the opposite end of the molecule or its insertion into the C1–C2 bond. Not counting thermal stabilization of the initial peroxy adducts, which prevails at low temperatures and high pressures, all other products share a minor yield of under 5%. Rate constants for the significant reaction channels have been fitted to modified Arrhenius expressions and are proposed for kinetic modeling of the oxidation of aromatic molecules and 1,3-butadiene. As a secondary reaction, n-C4H5 + O2 can be a source for the formation of acrolein observed experimentally in oxidation of the phenyl radical at low combustion temperatures, whereas another significant (secondary) product of the C6H5 + O2 reaction, furan, could be formed through unimolecular decomposition of 1-oxo-n-butadienyl. Both the n-C4H5 + O2 reaction and unimolecular decomposition of its 1-oxo-n-butadienyl primary product are shown not to be a substantial source of ketene.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...