Hydrogen adsorption on inorganic benzenes decorated with alkali metal cations: theoretical study†
Abstract
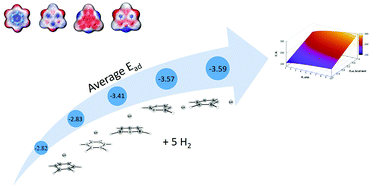
Hydrogen adsorption on different benzenes, both organic and inorganic, decorated with Li cations (Li+) was systematically studied by using quantum chemistry techniques. Our calculations demonstrate that Li+-decoration enhances the hydrogen storage ability of the complexes. MP2 calculations reveal that one to five hydrogen molecules per Li+ have high adsorption energies (Ead), up to −4.77 kcal mol−1, which is crucial for effective adsorption/desorption performance. The assessed hydrogen capacity of studied complexes is in the range of 10.0–10.6 wt%. SAPT2 calculations confirmed that induction and electrostatic interactions play the major role for H2 adsorption of the investigated systems, whereas London dispersion contributes to Ead moderately only in the cases of large number of hydrogen molecules adsorbed. Independent gradient model (IGM) analysis showed that there exists non-covalent bonding between Li+ and H2. The obtained van't Hoff desorption temperatures substantially exceed the temperature of liquid nitrogen. Ab initio molecular dynamics simulations confirmed the stability of the studied complexes. Our investigations establish the high potential of the studied complexes for usage in systems for hydrogen storage.


 Please wait while we load your content...
Please wait while we load your content...