Ionic-strength and pH dependent reactivities of ascorbic acid toward ozone in aqueous micro-droplets studied using aerosol optical tweezers†
Abstract
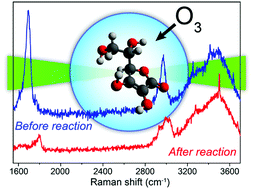
The heterogeneous oxidation reaction of single aqueous ascorbic acid (AH2) aerosol particles with gas-phase ozone was investigated in this study utilizing aerosol optical tweezers with Raman spectroscopy. The measured liquid-phase bimolecular rate coefficients of the AH2 + O3 reaction exhibit a significant pH dependence, and the corresponding values at ionic strength 0.2 M are (3.1 ± 2.0) × 105 M−1 s−1 and (1.2 ± 0.6) × 107 M−1 s−1 for pH ≈ 2 and 6, respectively. These results measured in micron-sized droplets are in agreement with those from previous bulk measurements, indicating that the observed aerosol reaction kinetics can be solely explained by liquid phase diffusion and AH2 + O3 reaction. Furthermore, the results indicate that high ionic strengths could enhance the liquid-phase rate coefficients of the AH2 + O3 reaction. The results also exhibit a negative ozone pressure dependence that can be rationalized in terms of a Langmuir–Hinshelwood type mechanism for the heterogeneous oxidation of AH2 aerosol particles by gas-phase ozone. The results of the present work imply that in acidified airway-lining fluids the antioxidant ability of AH2 against atmospheric ozone will be significantly suppressed.


 Please wait while we load your content...
Please wait while we load your content...