Theoretical and experimental study of peroxy and alkoxy radicals in the NO3-initiated oxidation of isoprene†
Abstract
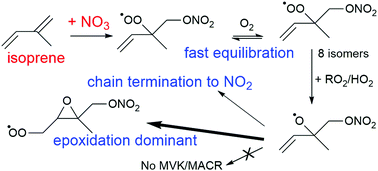
The initial stages of the nitrate radical (NO3) initiated oxidation of isoprene, in particular the fate of the peroxy (RO2) and alkoxy (RO) radicals, are examined by an extensive set of quantum chemical and theoretical kinetic calculations. It is shown that the oxidation mechanism is highly complex, and bears similarities to its OH-initiated oxidation mechanism as studied intensively over the last decade. The nascent nitrated RO2 radicals can interconvert by successive O2 addition/elimination reactions, and potentially have access to a wide range of unimolecular reactions with rate coefficients as high as 35 s−1; the contribution of this chemistry could not be ascertained experimentally. The chemistry of the alkoxy radicals derived from these peroxy radicals is affected by the nitrate moiety, and can lead to the formation of nitrated epoxy peroxy radicals in competition with isomerisation and decomposition channels that terminate the organic radical chain by NO2 elimination. The theoretical predictions are implemented in the FZJ-NO3-isoprene mechanism for NO3-initiated atmospheric oxidation of isoprene. The model predictions are compared against peroxy radical (RO2) and methyl vinyl ketone (MVK) measurements in a set of experiments on the isoprene + NO3 reaction system performed in the SAPHIR environmental chamber (IsopNO3 campaign). It is shown that the formation of NO2 from the peroxy radicals can prevent a large fraction of the peroxy radicals from being measured by the laser-induced fluorescence (ROxLIF) technique that relies on a quantitative conversion of peroxy radicals to hydroxyl radicals. Accounting for the relative conversion efficiency of RO2 species in the experiments, the agreement between observations and the theory-based FZJ-NO3-isoprene model predictions improves significantly. In addition, MVK formation in the NO3-initiated oxidation was found to be suppressed by the epoxidation of the unsaturated RO radical intermediates, allowing the model-predicted MVK concentrations to be in good agreement with the measurements. The FZJ-NO3-isoprene mechanism is compared against the MCM v3.3.1 and Wennberg et al. (2018) mechanisms.
- This article is part of the themed collection: 2021 PCCP HOT Articles


 Please wait while we load your content...
Please wait while we load your content...