Theoretical prediction by DFT and experimental observation of heterocation-doping effects on hydrogen adsorption and migration over the CeO2(111) surface†
Abstract
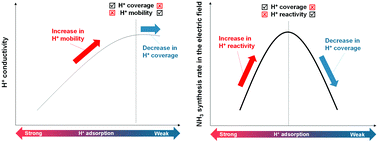
Hydrogen (H) atom adsorption and migration over the CeO2-based materials surface are of great importance because of its wide applications to catalytic reactions and electrochemical devices. Therefore, comprehensive knowledge for controlling the H atom adsorption and migration over CeO2-based materials is crucially important. For controlling H atom adsorption and migration, we investigated irreducible divalent, trivalent, and quadrivalent heterocation-doping effects on H atom adsorption and migration over the CeO2(111) surface using density functional theory (DFT) calculations. Results revealed that the electron-deficient lattice oxygen (Olat) and the flexible CeO2 matrix played key roles in strong adsorption of H atoms. Heterocations with smaller valence and smaller ionic radius induced the electron-deficient Olat. In addition, smaller cation doping enhanced the CeO2 matrix flexibility. Moreover, we confirmed the influence of H atom adsorption controlled by doping on surface proton migration (i.e. surface protonics) and catalytic reaction involving surface protonics (NH3 synthesis in an electric field). Results confirmed clear correlation between H atom adsorption energy and surface protonics.


 Please wait while we load your content...
Please wait while we load your content...