Reversible electrochemical conversion from selenium to cuprous selenide†
Abstract
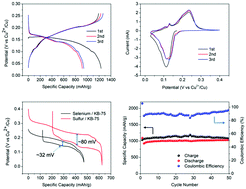
Using elemental selenium as an electrode, the redox-active Cu2+/Cu+ ion is reversibly hosted via the sequential conversion reactions of Se → CuSe → Cu3Se2 → Cu2Se. The four-electron redox process from Se to Cu2Se produces a high initial specific capacity of 1233 mA h g−1 based on the mass of selenium alone or 472 mA h g−1 based on the mass of Cu2Se, the fully discharged product.


 Please wait while we load your content...
Please wait while we load your content...