Reductive hydrobenzylation of terminal alkynes via photoredox and nickel dual catalysis†
Abstract
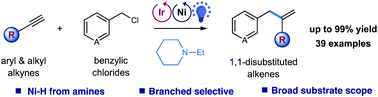
A photoredox/nickel dual catalyzed reductive hydrobenzylation of alkynes and benzyl chlorides by employing alkyl amines as a stoichiometric reductant is described. This synergistic protocol proceeds via Markovnikov-selective migratory insertion of an alkyne into nickel hydride, followed by cross-coupling with benzyl chloride, providing facile access to important 1,1-disubstituted olefins. This reaction enables the generation of nickel hydride by utilizing readily available alkyl amines as the hydrogen source. The mild conditions are compatible with a wide range of aryl and alkyl alkynes as well as chlorides.
- This article is part of the themed collection: 2021 Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...