Aerobic oxidation and oxidative esterification of alcohols through cooperative catalysis under metal-free conditions†
Abstract
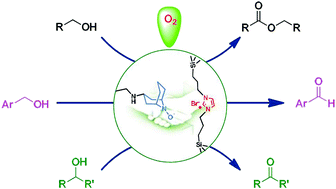
The ABNO@PMO-IL-Br material obtained by anchoring 9-azabicyclo[3.3.1]nonane-3-one N-oxyl (keto-ABNO) within the mesopores of periodic mesoporous organosilica with bridged imidazolium groups is a robust bifunctional catalyst for the metal-free aerobic oxidation of numerous primary and secondary alcohols under oxygen balloon reaction conditions. The catalyst, furthermore, can be successfully employed in the first metal-free self-esterification of primary aliphatic alcohols affording valued esters.
- This article is part of the themed collection: Green Synthesis


 Please wait while we load your content...
Please wait while we load your content...