Yb(iii)-catalysed syn-thioallylation of ynamides†
Abstract
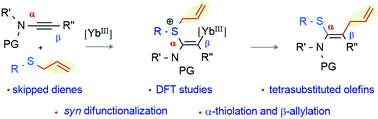
Reported herein is a syn-thioallylation of ynamides incorporating a sulfide moiety at the α-position and an allyl group at the β-position of the ynamide. The transformation is successful under ytterbium(III)-catalysis, providing access to highly substituted thioamino-skipped-dienes with broad substrate scope. Thus, tetrasubstituted olefins (with four different functional groups: amide, phenyl, thioaryl/alkyl, and allyl on the carbon centers) are made in a single step from readily accessible ynamides, preserving complete atom economy. The reaction can be extended to the synthesis of selenoamino dienes by ynamide syn-selenoallylation. DFT studies and control experiments provide insight into the reaction mechanism.


 Please wait while we load your content...
Please wait while we load your content...