Palladium-catalyzed cascade carbonylative annulation between alkene-tethered aryl iodides and carbon monoxide†
Abstract
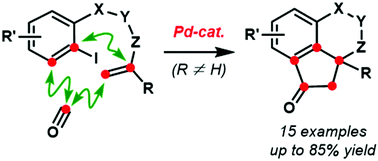
Rapid construction of molecules bearing all-substituted quaternary stereocenters represents a highly significant but challenging task in organic synthesis. Herein, we report a novel palladium-catalyzed cascade between alkene-tethered aryl iodides and carbon monoxide, which has resulted in a practical and powerful method for the synthesis of complex polycyclic molecules containing aryl-substituted quaternary stereocenters. Mechanistic studies suggested that the reaction proceeded via a Heck-type carbonylative cyclization, followed by a ketene-involved Friedel–Crafts acylation.


 Please wait while we load your content...
Please wait while we load your content...