Persistent 2c–3e σ-bonded heteronuclear radical cations centered on S/Se and P/As atoms†
Abstract
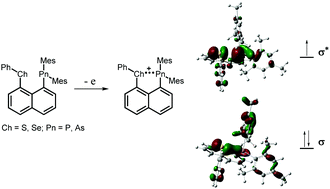
The two-center three-electron (2c-3e) bonded species are important in chemical and biological science. Reported isolable 2c-3e σ-bonded species are usually constructed in homoatomic radicals. The one-electron oxidation of main-group heteronuclear species Nap(SPh)(P(Mes)2) (1), Nap(SePh)(P(Mes)2) (2), Nap(SPh)(As(Mes)2) (3) and Nap(SePh)(As(Mes)2) (4) produced persistent radical cations 1˙+–4˙+ in solution. Large couplings of heteroatoms in EPR spectra of 1˙+–4˙+, shorter bond distances and bigger Wiberg bond orders of Ch–Pn in 1˙+–4˙+ than those in 1–4 in DFT calculations indicate large amounts of spin densities over heteroatoms and the formation of 2c-3e σ-bonds between chalcogen and pnicogen atoms. This work provides evidence of 2c-3e σ-bonds constructed between main-group heteronuclears and rare examples of radical cations involving three-electron σ-bonds between S/Se and P/As atoms.


 Please wait while we load your content...
Please wait while we load your content...