Biaryl sulfonamides as cisoid azosteres for photopharmacology†
Abstract
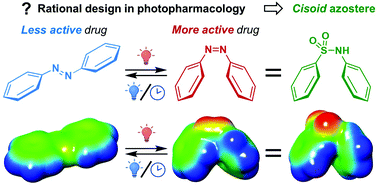
Biaryl sulfonamides are excellent candidates for the azologization approach that yields photoswitchable drugs more active in their metastable cis state, compared to the stable trans state. Here we present the scope and limitations of this strategy for rational design in photopharmacology.


 Please wait while we load your content...
Please wait while we load your content...