Enamides and dienamides in phosphoric acid-catalysed enantioselective cycloadditions for the synthesis of chiral amines
Abstract
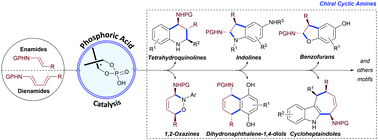
Chiral substituted cyclic amines are ubiquitous among biologically active molecules and natural products and are valuable intermediates in organic synthesis. Stable and easy to synthesize enamides and dienamides are versatile building blocks for the preparation of chiral amines. The exceptional synergy displayed between these synthetic synthons and chiral phosphoric acid catalysts has successfully been harnessed to develop straightforward formal cycloadditions exhibiting notably high enantiocontrol. This feature article showcases the remarkable versatility of these cycloadditions to access chiral cyclic amines with different ring sizes ranging from 5- to 7-membered rings, with an emphasis on biologically active natural products.


 Please wait while we load your content...
Please wait while we load your content...