Mass spectrometry reveals potential of β-lactams as SARS-CoV-2 Mpro inhibitors†
Abstract
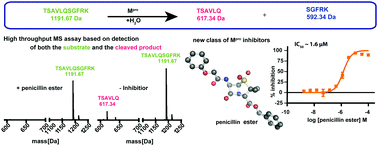
The main viral protease (Mpro) of SARS-CoV-2 is a nucleophilic cysteine hydrolase and a current target for anti-viral chemotherapy. We describe a high-throughput solid phase extraction coupled to mass spectrometry Mpro assay. The results reveal some β-lactams, including penicillin esters, are active site reacting Mpro inhibitors, thus highlighting the potential of acylating agents for Mpro inhibition.
- This article is part of the themed collection: Celebrating our 2021 Prizewinners


 Please wait while we load your content...
Please wait while we load your content...